This was a compulsory question. Majority of the candidates that answered
this question did fairly.
In part (a), majority of the candidates could not define ionic bond correctly.
Most of the candidates stated the type of bond existing in magnesium oxide as ionic/electrovalent but failed to state the bond in ammonium ion as covalent.
In part (b), some candidates were not able to determine the oxidation number
of sulphur in Na2S2O3. Many however did but carelessly omitted the positive
sign required for oxidation states.
In part (c), majority of the candidates defined Faraday’s first law of electrolysis
however, some candidates could not differentiate between quantity of electricity
and current thereby made mistake in their definitions.
In part (d), majority of the candidates gave examples of acid salt but many
candidates could not give example of basic salt.
In part (e), majority of the candidates could not identify the type of energy change
that occurred.
In part (f), majority of the candidates stated correctly the effect of Na2SO4(aq) on
litmus paper but many failed to realize the hydrolyzing effect of water of AlCl3 to produce an acidic solution and therefore stated that it is neutral to litmus thereby loosing the marks allocated to that question.
In part (g), some candidates gave the correct definition of efflorescent but most of
the candidates gave the definition for efflorescent substance instead.
In part (h), some of the candidates could not give uses of activated charcoal. However,
very few candidates were able to give the correct uses of activated charcoal.
In part (i), majority of the candidates were able to give one use for each of the stated processes.
In part (j), majority of the candidates were not able to calculate the amount in moles
of silver deposited by passing 10920C or electricity through a solution of silver salt.
The expected answers include:
- (a) (i) Ionic bond is the electrostatic force of attraction between
oppositely charged ions / cations and anions.
OR
Ionic bond is defined as the type of bond that is formed
from / by / through the transfer of electrons from one
atom of an element (metal) to an atom of another (non metal).
(ii) (I) - ionic / electrovalent
(II) - coordinate (covalent) / dative covalent
(b) (i) Na2S2O3
2 x Na + 2 x S + 3 x O = 0
2 x 1 + 2S + 3 (-2) = 0
2 + 2S – 6 = 0
2S = 4
S = +2
(c) Faraday’s first law states that the mass of an element discharged
during electrolysis is directly proportional to the quantity of
electricity passed
Accept equation Q 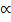 M where Q is quantity of electricity passed
M where Q is quantity of electricity passed
and M is mass deposited.
(d) (i) - NaHSO4, NaHCO3, KHSO3 etc
(ii) - Mg(OH)Cl, Zn(OH)Cl, Zn(OH)NO3 etc
(e) (i) - sublimation energy
(ii) - electron affinity
(f) (i) Na2SO4(aq) - No effect on litmus paper/neutral to litmus
(ii) AlCl3(aq) - Turns blue litmus paper red/acidic to litmus
(g) Loss of (all or part of) water of crystallization by a substance
on exposure to the air / atmosphere.
(h) - To remove colouring matter
- to adsorb gases.
- to remove impurities from liquids
- whitening of teeth
- to remove scent / to decolourize
(i) (i) Production of margarine / hardening of oils
(ii) Increases the yield of petrol / improves the quality of
petrol to produce raw materials or petrolchemicals
(iii) Production of alkanoates.
Accept esters are used
- as solvents for paints/gums/cellulose
- in perfumery
- in making cellulose acetate (for photographic films)
- as flavouring agents.
(j) Ag+(aq) + e- --------> Ag(s)
96500 C of electricity deposits 1 mole of Ag
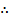 10920 C of electricity =
10920 C of electricity = 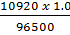
= 0.113 moles



