This question was popular among the candidates and their performance
was good.
In part (a), majority of the candidates define isotopes correctly. However,
some candidates defined isotopy instead of isotopes while others in their
definition of isotopes mentioned “elements” instead of “atoms or the
same elements”.
In part (b), majority of the candidates where able to write the electron configuration
of Mg and Al. However, some candidates could not correctly explain why the first
ionization energy or Mg is greater than that of Al.
Candidates could not satisfactorily give three different oxides of period 3 elements that react with water.
In part (c), majority of the candidates were able to define allotropes and name the crystalline allotropes of carbon.
In part (d), majority of the candidates could not list correct physical properties of the
gas evolved on warming crystals of NaCl with Conc. H2SO4. Some candidates could not write a correct balanced chemical equation for the reaction although a few did.
In part (e), most candidates could not convert 5.68mg to gram and therefore could not correctly calculate the amount of W in the microcomputer. However, a few did.
The expected answers include:
(a) (i) ion - electrically charged atom or group of
atoms formed from an atom by loss or gain of electron.
(ii) isotopes - atoms of the same element with the same
number of protons but different number of
neutrons / mass number.
(b) (i) I. 12Mg - 1s22s22p63s2
13Al - 1s22s22p63s22pl
II. The 3p electron in Aluminium is slightly further away
from the nucleus than the 3s electron of Magnesium.
The shielding/screening effect by the inner electrons is
more in Al than in Mg. These two effects outweigh
the increase in nuclear charge of Al, hence nuclear
attraction for 3s is greater than the 3p
OR
The 3s outermost orbital in Mg is fully filled and is
therefore more stable than the 3p orbital in Al which
is neither completely filled nor half filled and hence
less stable
OR
3s orbital of Mg is closer to the nucleus than the 3p
orbital of Al hence the nuclear attraction for the 3s is
greater than the 3p
(ii) Na2O, MgO, P4O10, SO3, Cl2O7, SO2, Na2O2, NaO2
(c) (i) Allotropes are different forms of the same element in the same
physical state.
(ii) Diamond and graphite
(iii) Diamond - for making jewellery
- for cutting hard surface materials
- making abrasives
Graphite - As a lubricant
- for making pencils
- for making electrodes
- neutron moderator
- making crucibles etc
(d) (i) - colourless
- has choking smell
- denser than air
- fumes in moist air
- soluble in water
(ii) 2NaCl + H2SO4 → Na2SO4 + 2HCl
OR
NaCl + H2SO4→NaHSO4 + HCl
(e) (i) Mass of Q = 5.68mg = 5.68 x 10-3g
Moles of Q = 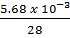
= 2.03 x 10-4 moles



