The question was poorly attempted by majority of the candidates.
In (a), only a few number of candidates were able to draw a labelled diagram of a simple electrolytic cell. Also, some candidates lost marks in (a)(iii) for giving formulae instead of names.
Question (c), was poorly attempted. Very few candidates could distinguish between cation in qualitative analysis. Most candidates could not distinguish the observation in using NaOH(aq) and NH3(aq).
In (d), candidates found it difficult to explain the term endpoint.
The expected answers include:
(a) (i)
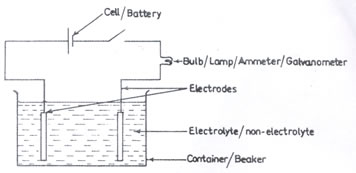
(ii) Electrolytic cell
(iii) I- Tetraoxosulphate (VI) acid
- sodium chloride solution
II - Glucose solution
- Sugar solution
- Petrol/Kerosene
(b) - Evaporating dish/Beaker
- Tripod stand and wire gauze
- Bunsen burner
- Burette/Pipette
- Glass rod / stirrer
- Thermometer
- Weighing balance/Scale
(c) (i) Ammonia solution
(ii) Ca2+ - No precipitate with NH3(aq)
Pb2+ - White chalky precipitate insoluble in excess
Zn2+ - White gelatinous precipitate soluble in excess
Al3+ - White gelatinous precipitate insoluble in excess
(d) End point is a stage/point in a titration where a given amount of reagent in a solution (acid oxidizing agent) completely react with another reagent (base reducing agent). It is usually indicated by colour change.



