|
Question 4
|
(a) Consider the following reaction scheme:

(i) Give the:
I chemical formula of each of compounds A,B,C,D and E
II IUPAC names of compounds A,B,C,D and E.
(ii) State the type of reaction involved in the conversion of CH3CH2CH20H to:
I. A;
II. B;
III. C.
[13 marks]
(b) (i) Write a balanced chemical equation for the reactions in of chlorine with [ 4 marks]
I ethane,
II ethene.
(ii) State the type of reaction exhibited in each of the reaction in 4(b)(i). [4 marks]
(c) (i) Give one product obtained from refining petroleum that is:
I gaseous;
II liquid;
III solid.
(ii) State one use of each of the products mentioned in 4(c)(i). [ 6 marks]
(d) Write a balanced chemical equation for the oxidation of glucose
(C6H1206) in living organisms.
[ 2 marks]
|
| _____________________________________________________________________________________________________ |
|
The question was popular among the candidates and the performance was fair.
In(a)(i) I-II, candidates could not give the chemical formula and IUPAC names of the
compounds represented as A,B,C,D and E in the scheme. The expected answers from
candidates were as follows:
(i) A - CH3CH2COOH
B - CH3 CH = CH2
C - CH3 CH2 COOCH2 CH3
o - CH3 CH2 COOH/CH3 CH2 OH
E - CH3 CH2 OH/CH3 CH2 COOH
(ii) A - propanoic acid
B - propene
C - ethyl propanoate
0- propanoic acid/ethanol
E - ethanol/propanoic acid
In(a)(iii).most candidates did not know that conversion to A is oxidation, B is dehydration and C
is esterification in I -III.
In part(b)(i)1 and II, candidates correctly wrote balanced equation for the reaction of chlorine
with ethane and ethene respectively thus:
I. C2H6 + Cl2 -'CH3 CH2 CI + HCL
II. CH2 = CH2 ----+ CH2 ClCH2 CL
In(b)(ii), candidates know that the type of reaction in (i) I and II were substitution and
addition, respectively.
In part(c), candidates correctly gave one product obtained from refining petroleum that is
gaseous, liquid and solid as butane/propane/ethane/methane/ethane,kerosene/dieselOil/lubricating oil/gasoline and asphalt/bitumen/paraffin wax respectively.
In part(d) only few candidates could write a balanced chemical equation for the oxidation of
glucose in living organisms thus:
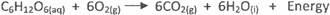
.
|
|
|
|



