Question 1
-
A is a solution containing 6.00 g of impure sodium trioxocarbonate (IV) in 1.00 dm3.
B is 0.100 moldm-3 hydrochloric acid. Titration of 25.0 cm3 portions of A with B using methyl orange as indicator gave the following results:Burette readings / cm3
1
2
3
Final reading
24.80
36.80
43.40
Initial reading
0.00
18.80
Volume of Bused
24.30
(a) (i) Complete the table.
[14 marks]
(ii) Calculate the average volume of B used.
The equation of the reaction is:
Na2CO3(aq) + 2HCl(aq)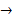 2NaCl(aq) + H2O(l) + CO2(g)
2NaCl(aq) + H2O(l) + CO2(g)
(b) From the information provided, calculate the:
(i) concentration, in moldm-3 of the sodium trioxocarbonate (IV) in A;
(ii) mass of sodium trioxocarbonate (IV) in 1.00 dm-3 of solution A;
(iii) percentage by mass of sodium trioxocarbonate (IV) in the impure sample.
[C = 12.0, O = 16.0, Na = 23.0]
Observation
This was a popular question among the candidates as majority of them responded to it.
In part (a), majority of the candidates mistook A for acid and B for base and this error affected their performance.
In part (b), few candidates were able to calculate the concentration, in moldm-3 of the sodium trioxocarbonate (IV) in A. in addition to this, they could not calculate the percentage by mass of sodium trioxocarbonate (IV) in the impure sample.
The expected answers include:
(a) (i)
Burette readings / cm3 |
1 |
2 |
3 |
Final reading |
24.80 |
36.80 |
43.40 |
Initial reading |
0.00 |
12.50 |
18.80 |
Volume of B used |
24.80 |
24.30 |
24.60 |
(ii) Average volume of B used =24.80 + 24.60
2
= 24.70 cm3
(b)
