Question 2
- Sample C is a sample organic compound that contains one or more of the following
functional group(s): alkene, alkanoic acid, alkanol or reducing sugar. The following tests were carried out on C.
Complete the table below.
|
Test |
Observation |
Inference |
(i) |
C + ……………. |
Reddish-brown |
……………………… |
(ii) |
C + Na2CO3(aq) |
……………………… |
Gas is CO2 |
(iii) |
C + ZnCl2(aq) |
no two layers formed
|
…………………… |
(iv) |
C + ……………… +heat |
no brick-red precipitate formed |
………………………. |
[10 marks]
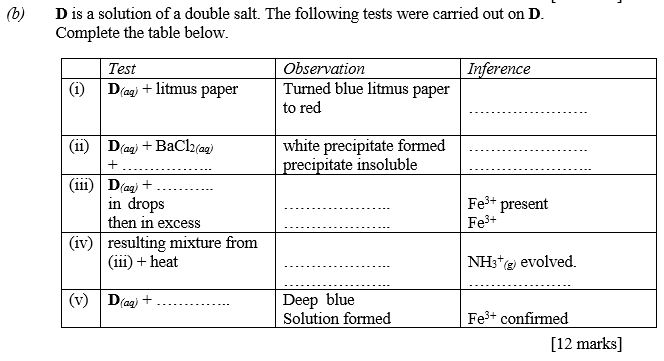
(b) D is a solution of a double salt. The following tests were carried out on D.
Complete the table below.
[12 marks]
Observation
This question tested the knowledge of qualitative analysis. Majority of the candidates responded to this question. However, their performance was below average.
In part (a), majority of the candidates could not write not write the correct reactants under test. Also, they could not write the observation and inference.
In part (b), majority of the candidates could not write not write the correct reactants under test. Also, they could not write the observation and inference.
The expected answers include:
(a)
a |
Test |
Observation |
Inference |
(i) |
C + Br2 / CCl4 |
Reddish-brown colour not discharged. |
Alkene group absent |
(ii) |
C + NaHCO3(aq) |
(Effervescence), colourless, odourless gas evolved gas turned lime water milky |
Gas is CO2 |
(iii) |
C + ZnCl2(aq) |
No two layers formed |
Alkanol group absent |
(iv) |
C + Fehlings soln |
No brick – red precipitate formed |
Reducing sugar absent |
(b)
b |
Test |
Observation |
Inference |
(i) |
D(aq) + litmus paper |
Turned blue litmus paper to red |
D is |
(ii) |
D(aq) + BaCl2(aq) + dilHCl |
White precipitate formed Precipitate insoluble |
, , present |
(iii) |
D(aq) + NaOH(aq) in drops (Accept NH3(aq)) |
Reddish brown gelatinous precipitate is formed |
Fe3+ present |
(iv) |
Resulting mixture from (iii) + heat |
Pungent/choking/irritating colourless gas evolved/produce dense white fumes with HCl |
evolved present |
(v) |
D(aq) + [K4Fe(CN)6](aq) (1) (Accept IUPAC name) |
Deep blue solution formed |
Fe3+ confirmed |
