Question 9
-
- State four effects of heat on substances.
- Explain the statement latent heat of fusion of ice is 3.34 x 105 J kg-1
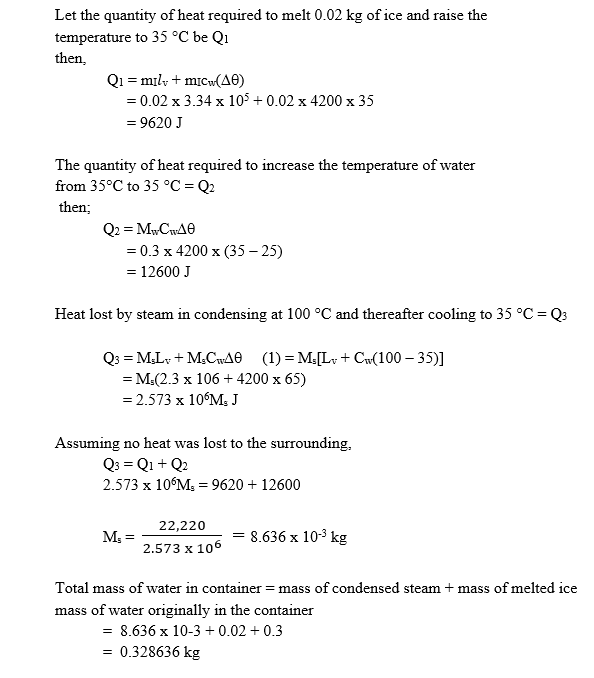
- A mass of 0.3 kg of water at 35oC and 0.02 kg of ice at 0oC are put in a lagged container. Steam at 100 oC is then passed into the mixture until all the ice melts and the final temperature of the mixture is 35oC. Calculate the mass of water in the container at 35oC. [Specific latent heat of vaporization of steam is 2.3 x 106 J kg,-1, specific latent heat of fusion of ice is 3.34 x 105 J kg-1, specific heat capacity of water is 4200 J kg-1 K-1].
Observation
Part (a) Most candidates could not explain specific latent heat of fusion in relation to ice.
Part (b) Many candidates this not attempt this part. Performance was below average
The expected answer is:
-
-
Effects of heat on substances
- Increase in temperature
- Thermal expansion
- Decrease in resistance of semiconductors
- Change of state
- Thermionic emission
- Increase in resistance of metals
- Reduction in viscosity of liquids
- Increase in viscosity of gases
-
Explanation of statement ‘the specific latent heat of fusion of ice
is 3.34 x 105 J kg-1
It means that the quantity of heat required to change
1 kg of ice at 0 °C to water at 0 °C is 3.34 x 105 J
-
Effects of heat on substances
-
Calculation of required mass of water