Question 5
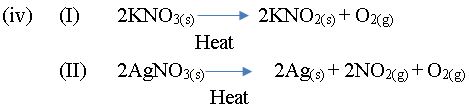
- KNO3(s);
. (a) (i) Determine the oxidation number of sulphur in Na2S2O3.
(ii) Name the allotropes of sulphur.
(iii) State two ways in which the structure of graphite and diamond
are similar. [6 marks]
(b) (i) Name two green-house gases.
(ii) State one effect of an increased level of green-house gases on the
environment.
(iii) State one source from which nitrogen (1) oxide is released into the environment.
(iv) Write a chemical equation to show the effect of heat on each of the following compounds:
II. AgNO3(s). [8 marks]
(c) (i) Describe briefly how pure dry crystals of calcium chloride could be
obtained from a solution of calcium chloride.
(ii) Explain briefly each of the following observations:
I. ammonia gas is highly soluble in water;
II. boiling point of chlorine is lower than that of iodine. [7 marks]
(d) Consider the reaction represented by the following equation:
![]()
Calculate the volume of HCl gas that can be obtained at s.t.p. from
5.85 g of sodium chloride.
[Na = 23.0, Cl = 35.5, Molar volume of gas at s.t.p. = 22.4 dm3] [4 marks]
Observation
The question was popular, and majority of the candidates responded to it.
In part (a), majority of the candidates calculated the oxidation number of sulphur in Na2S2 03,
and were familiar with the allotropes of sulphur.
In part (b), majority of the candidates were not familiar with green house gases, but could give the source from which nitrogen (I) oxide is released into the environment. Candidates could not write a chemical equation to show the effect of heat on KNO3(s) and AgNO3(s).
In part (c), majority of the candidates could not outline the procedures for obtaining pure dry crystals of calcium chloride from a solution of calcium chloride. In addition, they could not explain why ammonia gas is highly soluble in water. However, majority of the candidates explained why the boiling point of chlorine is lower than that of iodine.
In part (d), majority of the candidates showed poor knowledge of mass/volume relationships in chemical reactions.
The expected answers include:
(a) (i) let x = oxidation number of sulphur
2(1) + 2x + 3(-2) = 0
x = +2
(ii) allotropes of Sulphur are:
- rhombic / alpha sulphur
- monoclinic / Beta / prismatic sulphur
- amorphous / delta sulphur
- plastic sulphur
(iii) - Macromolecule / giant molecule/3 dimensional
- carbon atoms are covalently bonded
- crystalline in nature
(b) (i) - Carbon (IV) oxide
- methane
- nitrogen (1) oxide
- Water vapour
- ozone
- Chlorofluorocarbons
(ii) - Global warming / climate change
(iii) - Ammonium trioxonitrate (V)/aerosol
(Accept ammonium nitrate)
- Combustion of fuel at high temperature
(c) (i) Heat the solution to concentrate it/leave in a warm place / partially
evaporate solution and allow to cool filter off crystals / pick out crystals
Dry crystals with filter paper
(ii) (I) Ammonia readily associates with water molecules through
hydrogen bonding
(II) Chlorine molecules are smaller /chlorine molecules have fewer number
of electrons and the strength of van der waal’s
forces between molecules is weaker as compared to that of
iodine.
OR
Iodine molecules are bigger and the vander Waals forces between
molecules are stronger as compared to that of chlorine.
(d) Mr(NaCl) = 23.0 + 35.5
= 58.5 g/mol
Mass of NaCl = 5.85g
