Question 1
1. (a) Explain briefly why 42He has a stable electron configuration compared to 94Be [2 marks](b) Consider the following elements: 1H and 3Li.
(i) State the number of electrons that an atom of each element would have after forming an ionic bond.
(ii) Give a reason for each of your answers stated in 1(b)(i). [4 marks]
(c) State two factors that should be considered when siting a chemical industry. [2 marks]
(d) State two advantages of using a catalyst instead of high temperatures in chemical reactions.[2 marks]
(e) Turpentine burns in chlorine according to the following equation:
C10H16(I) + 8Cl2(g) → 10C(s) + 16HCl(g)
Calculate the mass of turpentine that would completely burn in 21.3 g of chlorine.
[Molar mass of chlorine = 71 gmol-1; Molar mass of Turpentine = 136 gmol-1]
(f) What is cracking? [2 marks]
(g) State two factors that may influence the value of electron affinity. [2 marks]
(h) What are carbohydrates? [2 marks]
(i) State two differences between a simple sugar and starch. [2 marks]
(j) Write an equation to show the dissociation of each of the following acids:
(i) H2CO3;
(ii) CH3COOH. [2 marks]
Observation
This question was a compulsory question. Almost all the candidates responded to it.In part (a), majority of the candidates could not explain why 42He has a stable electron configuration compared to 94Be
In part (b), few candidates could state the number of electrons that an atom of each element would have after forming an ionic bond.
In part (c), about 70% the candidates stated the factors that should be considered when siting a chemical industry.
In part (d), majority of the candidates could not state the advantages of using a catalyst instead of high temperatures in chemical reactions.
In part (e), majority of the candidates calculated the mass of turpentine that would completely burn in 21.3 g of chlorine.
In par (f), about 95% of the candidates were able to explain the term cracking.
In part (g), few candidates stated two factors that may influence the value of electron affinity.
In part (h), majority of the candidates could not explain carbohydrates;
In part (i), majority of the candidates were able to differentiate between a simple sugar and starch.
In part (j), majority of the candidates were able to show the dissociation of H2CO3 and CH3COOH.
The expected answers include:
(a) 42HeHe has its outermost / valence shell fully filled with electrons/ has maximum number of electrons in its outermost shell/ duplet thus more stable whereas 94Be has incompletely filled outermost / valence shell hence less stable.
(b) (i) 1H - 2 electrons / zero
3Li - 2 electrons
(ii)1H - it accepts one electron / donates one electron
3Li - it loses an electron to have a duplet shell
(c) - nearness to raw material / feed stock
- nearness to markets
- labour supply
- transportation
- availability of power supply
- government policy
- away from residential areas
- storage facilities for raw materials
- source of water supply
- conducive climate
(d) - saves money / the plant does not have to operate for long to produce
- reduces energy / reaction proceeds at a much lower temperature
- no undesirable products are formed / catalyst is specific in action
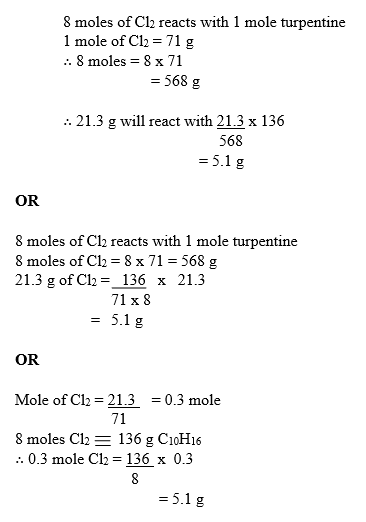
(e) From the equation:
8 moles of Cl2 reacts with 1 mole turpentine
(b) (i) 1H - 2 electrons / zero
3Li - 2 electrons
(ii)1H - it accepts one electron / donates one electron
3Li - it loses an electron to have a duplet shell
(c) - nearness to raw material / feed stock
- nearness to markets
- labour supply
- transportation
- availability of power supply
- government policy
- away from residential areas
- storage facilities for raw materials
- source of water supply
- conducive climate
(d) - saves money / the plant does not have to operate for long to produce
- reduces energy / reaction proceeds at a much lower temperature
- no undesirable products are formed / catalyst is specific in action
(e) From the equation:
8 moles of Cl2 reacts with 1 mole turpentine
(f) Cracking is the breaking down of long chain hydrocarbons into smaller molecules by the action of heat and / or in the presence of a catalyst.
(g) - nuclear charge
- atomic size
- electron configuration
(h) Carbohydrates are molecules / organic compounds consisting of carbon, hydrogen and oxygen atoms usually with hydrogen and oxygen atoms in the ratio of 2:1.
Sugar |
Starch |
-monosaccharides / low molar Mass |
- polysaccharides / high molar mass |
- single units of specific molecules |
- long chains of single sugar molecule/ subunits linked together |
- general formula CxH2yOy |
- general formula (C6H10O5)n |
- crystalline |
- amorphous / non crystalline |
-soluble in water |
-insoluble in water |
