Question 2
(a) (i) Name three different methods for preparing salts.(ii) How does the theory explain the law of multiple proportion? [11 marks]
(iii) State two uses of sodium trioxocarbonate (IV). [11 marks]
(b) If you were given some impure copper, describe how you would obtain a specimen of the pure metal by electrolysis. [6 marks]
(c) Given that sodium chloride has a solubility of 36.3 at 30 ℃ and 39.0 at 100 ℃ and that of silver nitrate is 297.0 at 30 ℃ and 952.0 at 100 ℃.
(ii) Deduce which of the two salts can be purified more efficiently by crystallization.[8 marks]
Observation
This question was popular among the candidates. About 70% of them responded to it.In part (a), majority of the candidates were able to name three different methods of preparing salts. However, only few of them could give example of a balanced equation for each of the named methods.
In part (b), few of the candidates were able to describe how a specimen of pure metal could be obtained from impure copper by electrolysis.
In part (c), majority of the candidates were able to calculate the percentage of each of the given substances in the saturated solution at 100 ℃ that is deposited on cooling to 30 ℃.
The expected answers include:
(a) (i) - action of an acid upon an active metal / metals higher than hydrogen in the reactivity series
- preparation by double decomposition (reaction between two soluble compounds to produce an insoluble compound)
- by neutralization (reaction between acids & bases)
- action of an acid on trioxocarbonate (IV) of a metal
- direct combination of elements
- action of dilute acid on an insoluble base
Examples:
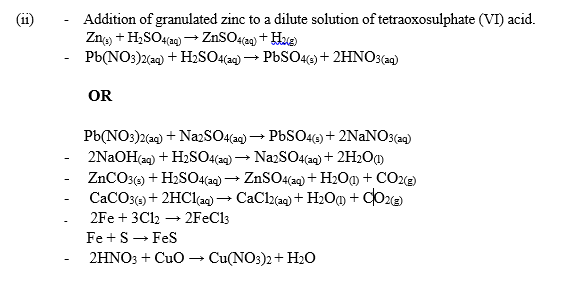
(iii) Uses of Na2CO3
- used as a water softner
- manufacture of glass/paper/rayon/soaps/detergents/NaOH/Borax
- food processing aid
- pH modifier
- swimming pool chemical
- electrolyte
- analytical reagent / standardization of acids
- preparation by double decomposition (reaction between two soluble compounds to produce an insoluble compound)
- by neutralization (reaction between acids & bases)
- action of an acid on trioxocarbonate (IV) of a metal
- direct combination of elements
- action of dilute acid on an insoluble base
Examples:
(iii) Uses of Na2CO3
- used as a water softner
- manufacture of glass/paper/rayon/soaps/detergents/NaOH/Borax
- food processing aid
- pH modifier
- swimming pool chemical
- electrolyte
- analytical reagent / standardization of acids
(ii) AgNO3 (Silver trioxonitrate (V)) will be more efficiently crystallized because it has a higher percentage deposited.
