Question 9
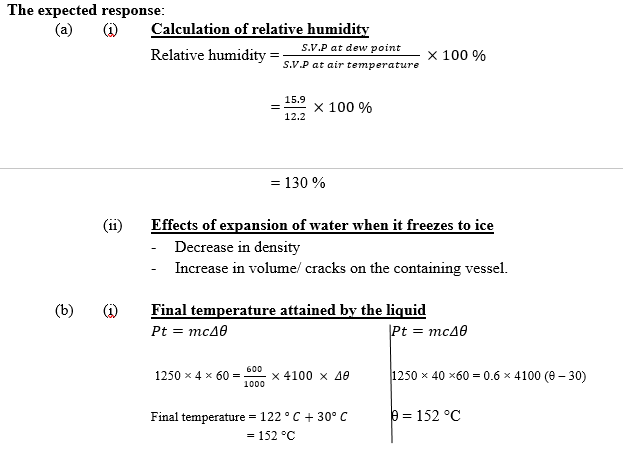
(a) (i) The s.v.p. of water vapour on acertain day at air temperature 28 0C is 12.2 mmHg. Calculate the relative humidity of a day when the s.v.p. of water vapour at dew point is 15.9 mmHg.
(ii) State two effects of expansion of water when it freezes to ice.
(b) A quantity of liquid of mass 600 g placed in a plastic container with a tight fitting lid has a temperature of 30 0C. The container is placed in a microwave oven rated 1250 W.
(i) if the microwave is operated for 4 minutes, calculate the final temperature attained by the liquid. (assuming no heat losses [Specific heat capacity of the liquid = 4100 j kg-1 K-1]
(ii) If the liquid is brought out and allowed to cool, a dent is observed on the container. Explain.
(c) Explain why containers with tight-fitting lids are not suitable for use in microwave cooking.
(d) (i) Define fixed points on a temperature scale.
(ii) State one effect of heat on a substance.
Observation
 (ii) The container is dented because the condensation of the steam will lead to a decrease in pressure making the atmospheric/external pressure greater than the pressure in the container.
(ii) The container is dented because the condensation of the steam will lead to a decrease in pressure making the atmospheric/external pressure greater than the pressure in the container.
(c) Explanation of observation
- The molecules of the steam are confined. As the temperature increases, the rate of
- collision of the molecules with walls of the container increases, the pressure
- inside the container increases, causing it to burst.
(d) (i) Fixed point on a temperature scale
Accurate and standard temperatures used as reference point on the temperature scale.
(ii) Effects of heat on a substance:
- Change:
- in temperature
- in (electrical) resistance
- of state
- in colour
- in dimension
