Question 2
- Fig. 1 is an atomic structure.
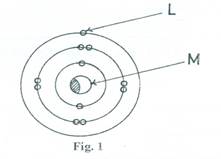
- Identify the parts labeled L and M.
- State one property of the atom as determined by L.
- Name the particles contained in M.
- Fig. 2 shows two particles attracting each other.
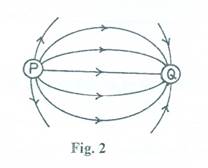
State the:
- charges possessed by each of particles P and Q.
- effect of taking Q far away from P.
- illustrate with the aid of a diagram, the effect of replacing Q with another P in Fig. 2.
Observation
The expected answers were:
(a) (i) L – Electron
M – Nucleus
(ii) Properties of the atom determined by L
- It is an atom of a metal/conductor
- It is a good conductor of electricity and heat.
- It has one valence electron
(iii) Proton and neutron
(b) (i) P –positive charge (or +);
Q – negative charge ( or - )
(ii) No/weak attraction
(c) Diagram of effect of replacing Q with another P
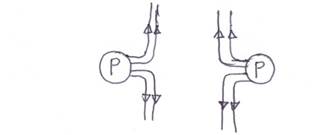
Majority of the candidates that attempted this question responded well.
