This was a popular question and most candidates did well in some parts. Part (a), candidates definition of solubility was faulty as few of them related it to solvent instead of solution. Some even defined saturated solution and not solubility.
Part (b), most candidates were able to draw correctly the graph required. However, few candidates did not distinguished between the axes, some even interchange the labelling of the axes, some chose wrong scales. The calculation was poorly handled.
In part (c), equation of reactions could not be written correctly by most candidates. Candidates lack the knowledge of what are produced and their formulae. However, most candidates were able to answer correctly (c)(ii) and (iii).
Part (d), many candidates were able to determine the oxidation state of Al in [Al(H2O)6]3+ and H in NaH. However, some candidates did not indicate a sign on their answer.
The expected answers are:
(a) Solubility is the maximum amount of solute that would dissolve/be contained in 1 dm3 of
solution at a given temperature.
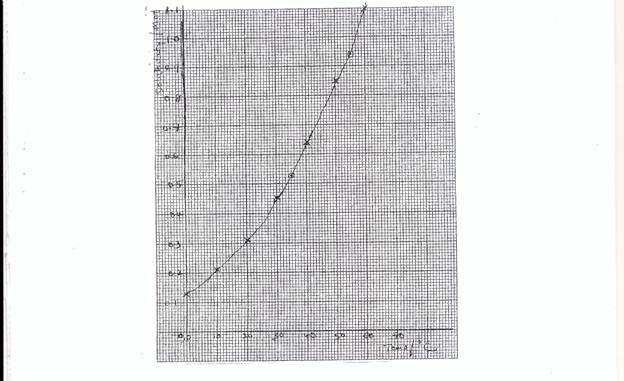
- suitable and reasonable scale. – Graph should cover ¾ of the page
- 4 points correctly plotted
- correct shape of curve
- axes
(ii) At 35oC solubility = 0.50 moldm-3 range
(iii) Solubility at 55oC = 0.95 to 0.98 moldm-3 range say X 1 moldm-3 range
say X 1 moldm-3 of salt Z = 0.97 x 100
= 97g
∴ 100 cm3 will contain = 97 x 100
1000
= 9.7g
Solubility at 35oC = 0.54 moldm-3
1 dm3 will contain = 0.54 x 100g
= 54 g
Mass of salt that would crystallize out
9.7 – 5.4 = 4.3g
(c) (i) I. Al2O3(S) + 6HC(aq) → 2AlCl3(aq) + 3H2O(1)
II. Al2O3(S) + 2NaOH(aq) + 3H2O(1) → 2NaAl(OH)4
(ii) amphoteric
(iii) ZnO, PbO, SnO, A Al2O3
(d) (i) I. [Al(H2O)6]3+ X + (0) = + 3
X = + 3
Al = + 3
II. NaH = 1 + X = 0 (1)
X = -1
H = -1
(ii) I. Copper (II) tetraoxosulphate (VI) pentahydrate
II. Calcium trioxocarbonate (IV)
III. Potassium tetraoxomanganate (VII)



