A good number of candidates attempted this question and the performance was fair.
In part (a), majority of the candidates were able to give the definition of functional
group correctly. However, some candidates could not identify the functional group
in the structure.
In part (b), majority of the candidates could not state the type of reaction that Q
would undergo with Cl2. Also, most of the candidates could not draw the structure or
the alkene that is an isomer of compound Q.
In part (c), majority of the candidates that attempted this part could not state the reason
why B gave white precipitate with ammoniacal AgNO3 solution. Only a few
candidates gave the name of the reaction between C and D as esterification.
In part (d), majority of the candidates could not describe the production of biogas using
a biogas generator. However, some were able to give uses of biogas.
The expected answers include:
(a) (i) Functional group is a bond, an atom or group of atoms which
give an organic compound its characteristic chemical properties
(ii) alkanol
alkanoate
(b) (i) Cyclopropane
(ii) 2C3H6 + 9O2 → 6CO2 + 6H2O
(iii) Substitution reaction
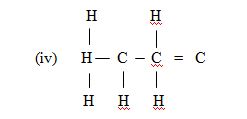
(c) (i) Sample A = alkene
Sample B = alkyne
Sample C = alkanol
Sample D = alkanoic acid
(ii) Both samples A and B are unsaturated compounds
(iii) Sample B is a terminal alkyne
(iv) Esterification
(v) - as a catalyst
- to remove water
Accept dehydration
(d) (i) The organic waste is fed into a biodigester where
different kinds of bacteria convert the decaying
matter into biogas CH4 / CO2 / H2 / CO through
the process of anaerobic decomposition.
(ii) - for cooking/fuel
- for heating/fuel
- for lighting/fuel
- as residue for fertilizer
- generating electricity 


