Question 1
(a) (i) Define an acid according to the Lewis concept.
(ii) Give one example of a Lewis acid. [3 marks]
(b) Explain salting out in soap preparation. [2 marks]
(c) State the reagent and condition necessary for the following conversion:
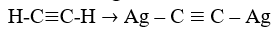 [2 marks]
[2 marks]
(d) What is the percentage abundance of an isotope? [2 marks]
(e)
(i) Why does the element with atomic number 18 not have an oxide?
(ii) Explain why chlorine(I) oxide has a low melting point.
[3 marks]
(f) Describe a test to distinguish between concentrated HNO3 and concentrated H2SO4.
[3 marks]
(g) State two differences between an electrochemical cell and an electrolytic cell.
[2 marks]
(h) How does the trend in ionization energy affect the reactivity of group I element?
[3 marks]
Define the term molecular formula
. [2 marks]
(j) (i) State which of the gases H2 and NH3 would deviate more from ideal behaviour.
(ii) Give reasons for the answer stated in 1(j)(i). [3 marks]
Observation
This question was popular among the candidates and majority of them responded to it.
In part (a), this question was deleted because it was not in Nigerian syllabus.
In part (b), majority of the candidates explained salting out in soap.
In part (c), majority of the candidates could not state the reagent and condition necessary for converting H-C.C -H Ag-C.C -Ag.
In part (d), few candidates were able to determine the percentage abundance of an isotope.
In part (e), majority of the candidates gave a reason why the element with atomic number 18 not have an oxide but majority of the candidates explained why chlorine(I) oxide has a low melting point.
In part (f), majority of the candidates could not describe a test to distinguish between concentrated HNO3 and concentrated H2SO4.
In part (g), majority of the candidates stated the differences between an electrochemical cell and electrolytic cell.
In part (h), majority of the candidates stated how the trend in ionization energy affects the reactivity of group I elements.
In part (i), majority of the candidates defined the term molecular formula.
In part (j), majority of the candidates could not state which of gases H2 and NH2 would deviate more from ideal behaviour.
The expected answers include:
(a) Question deleted
(b) Is the addition of NaCl to the reaction mixture in soap production to facilitate the precipitation of soap from the mixture
(c) reagent - AgNO3 in NH3(aq) / Ammoniacal silver trioxonitrate (V)
Condition - heat
(d) Is the fraction of a given isotope in a mixture of isotopes of the same element
OR
The % of an isotope with a specific atomic mass found in a naturally accruing sample of an element
(e) (i) because the element is /has
- unreactive
- stable
- inert
- a noble gas
- completely filled outermost shell
(ii) because of weak forces between the molecules hence not much heat is needed to break the forces
(f) - (Conc) HNO3 produces brown fumes (of NO2) when copper tunings are Added to it / copper (II) salt is added but H2SO4 will not react with the copper tuning / copper (II) salt.
- When Conc. H2SO4 is added to sugar it chars the sugar but Conc. HNO3 will not
- Add BaCl2 solution to each acid white precipitate indicates H2SO4
no precipitate indicates HNO3
(g)
Electrochemical cell |
Electrolytic cell |
- the anode is negative / cathode is |
- anode is positive / cathode is negative |
- chemical energy is converted to |
- electrical energy is converted to
|
- the two half cells are in separate |
- the two half cells are in the same |
- reaction is spontaneous |
- non- spontaneous |
- current is generated from within the |
- current is generated from external source / |
(h) group 1 elements reacts by forming positive ions, the higher the ionization energy, the more difficult it is to lose the electron hence the lower the reactivity of the
elements
OR
Ionization energy decreases down group 1 elements, the lower the ionization energy, the easier to loose an electron
(b) Is the addition of NaCl to the reaction mixture in soap production to facilitate the precipitation of soap from the mixture
(c) reagent - AgNO3 in NH3(aq) / Ammoniacal silver trioxonitrate (V)
Condition - heat
(d) Is the fraction of a given isotope in a mixture of isotopes of the same element
OR
The % of an isotope with a specific atomic mass found in a naturally accruing sample of an element
(e) (i) because the element is /has
- unreactive
- stable
- inert
- a noble gas
- completely filled outermost shell
(ii) because of weak forces between the molecules hence not much heat is needed to break the forces
(f) - (Conc) HNO3 produces brown fumes (of NO2) when copper tunings are Added to it / copper (II) salt is added but H2SO4 will not react with the copper tuning / copper (II) salt.
- When Conc. H2SO4 is added to sugar it chars the sugar but Conc. HNO3 will not
- Add BaCl2 solution to each acid white precipitate indicates H2SO4, no precipitate indicates HNO3
(g)
Electrochemical cell |
Electrolytic cell |
- the anode is negative / cathode is |
- anode is positive / cathode is negative |
- chemical energy is converted to |
- electrical energy is converted to |
- the two half cells are in separate |
- the two half cells are in the same |
- reaction is spontaneous |
- non- spontaneous |
- current is generated from within the |
- current is generated from external source / |
(h) group 1 elements reacts by forming positive ions, the higher the ionization energy, the more difficult it is to lose the electron hence the lower the reactivity of the
elements
OR
Ionization energy decreases down group 1 elements, the lower the ionization energy, the easier to lose an electron hence the higher the reactivity of the elements
(i) Molecular formula shows the actual number of atoms of each element in a molecule
(j) (i) NH3 will deviate more
(ii) because it has a larger volume and also it has a stronger intermolecular force hence the higher the reactivity of the elements
