Question 5
(a) (i) With the aid of an equation, explain briefly why aluminum metal is not affected by air.
(ii) In the extraction of aluminum from bauxite, state the:
- Substance used for purifying the ore;
- Composition of the mixture electrolyzed.
[8 marks]
(b) ZnO is an amphoteric oxide. Write equations to illustrate this statement.
[4 marks]
(c) (i) List three uses of sodium trioxocarbonate(IV).
(ii) Explain briefly why a solution of trioxnitrate(V) acid turns yellowish on storage for sometime.
(i) Describe briefly how trioxonitrate(V) ions could be tested for in the laboratory.
[8 marks]
(d) (i) Write balanced chemical equations for the preparation of hydrogen chloride;
- using concentrated H2SO4
- by direct combination of its constituent elements.
(ii) State one use of hydrogen chloride.
[5 marks]
Observation
This question was popular among the candidates as most of them responded to it.
In part (a), majority of the candidates described the observation that would be made when sulphur is heated from room temperature till 119 ºC.
In part (b), majority of the candidates stated two gaseous pollutants that can be generated by burning coal but could not state the gas responsible for most of the explosions in coal mines.
In part (c), majority of the candidates were able to describe a chemical test for water and were able to state the effect of boiling on a temporary hard water.
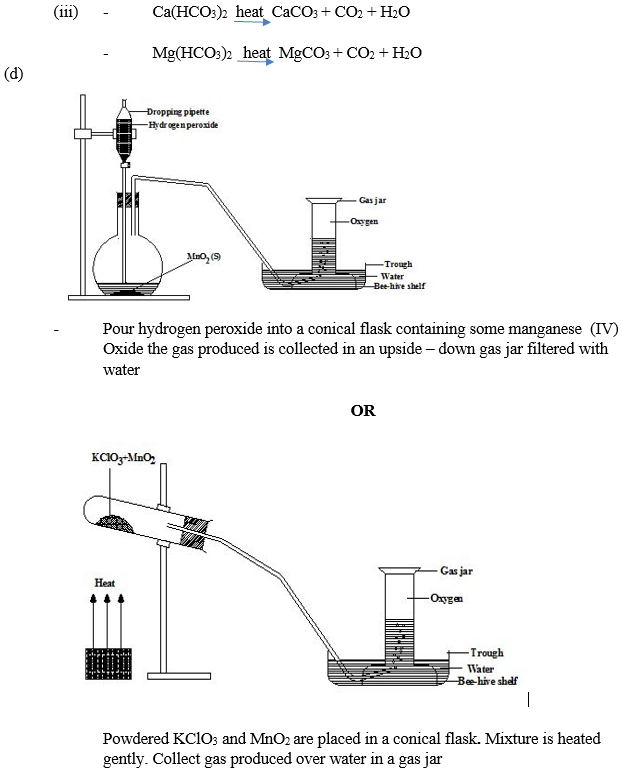
In part (d), majority of the candidates could not describe the laboratory preparation of oxygen gas with the aid of a labelled diagram.
The expected answers include:
(a) (i) Its colour darkens from pale yellow to amber colour while its crystals
become needle-like and at 119ºC it begins to melt
(ii) the solution turns green, bubbles of colourless gas rise from the bottom of
the mixture, but brown fumes of NO2 are produced and the solution later turns blue
(b) (i) - SO2
- NO2
- CO
- CO2
- H2S
(ii) methane (CH4)
(iii) - contamination of soil
- contamination of ground water
- soil erosion
- loss of biodiversity
- formation of sink holes
(iv) Because of the large surface area and the higher the area the faster the rate
of reaction
- Coke
(c) (i) the sample is added to anhydrous copper (II) tetraoxosulphate (VI) when it
turns from white to blue confirms the presence of water / sample is added to anhydrous cobalt chloride / cobalt chloride paper when it turns from blue to pink indicates the presence of water.
(ii) I. it softens temporary hard water
II. to remove permanent hardness / soften hard water