(a) (i) Define saturated solution.
(ii)The solubility of KN03 at 20°C was 3.00 mol dm-3 If 67.0g of KN03 was added to
250cm3 of water and stirred at 20°C, determine whether the solution formed was
saturated or not at that temperature.
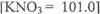 [7 marks]
[7 marks]
(b) (i) Distinguish between
dative bond and
covalent bond.
(ii) Explain why sugar and common salt do
not conduct electricity in the solid state.
(iii) State the type of intermolecular forces present in:
1. hydrogen fluoride;
II. argon.
(iv) Consider the compounds with the following structures:
S - H ----N and 0 - H -----N
In which of the compounds is the hydrogen bond stronger? Give reason for
your answer.
[ 12 marks]
(c) (i) State
Dalton's Law of Partial Pressure.
(ii) If 200cm
3 of carbon(IV) oxide were collected over water at 18°C and 700 mmHg,
determine the volume of the dry gas at s.t.p.
[ standard vapour pressure of water at 18°C = 15 mmHg] [6 marks]



