The question was the least popular among candidates and the performance was poor.
In (a)(i), most of the candidates who attempted the question could not explain why water was
referred to as universal solvent. They did not know that because it is polar, it can dissolve ionic
solute as well as partially ionic/polar covalent substances.
In (a)(ii), candidates were able to give one chemical test for water thus:
Add water to anhydrous copper(II) tetraoxosulphate (VI), salt colour changes from white to blue
OR
Add water to anhydrous cobalt(II) chloride, colour changes from blue to pink.
In part (b)(i) and (ii), candidates could not write balanced equation for the oxidation half
reaction, reduction half reaction and overall reaction nor calculate the volume of gas at the anode
at s.t.p.
The expected responses from candidates were as stated below:
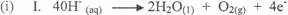
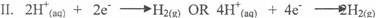
Ill. 2H20(l) → 2H2(g) + 02(g)
40H- (aq) + 4H+ (aq) →'2H2O(l) + 2H2(g) + 02(g)
(ii) Quantity of electricity = 1 x t
= 1.25 x 40 x 60
= 3000C
For 1 mole of O2 formed at the anode, 4e- are generated
Volume of O2 formed by 3000e = 1000 x 1 mole O2
96500 4
= 7.77 X 10-3 moles
1 mole of 02 occupies 22.4 dm3 at s.t.p
:. mole of 02 = 7.77 X 10-3 x 22.4 dm3
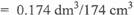
OR
From the equation at anode,
4 x 96500C = 22.4 dm3
3000C = 22.4 x 3000
4 x 96500
0.174dm3/174cm3
In part (c)(i), candidates could not balance the given chemical equation. The expected balanced
chemical equation from candidates was
2Au(s) + 3CL2(g) →2AuCl3(s)
In part (c )(ii), only very few candidates could determine which of the reactants was in excess and
the excess amount in I and II. The expected answers from candidates were as follows:
(ii) Amount of Au = 1.25
197
0.00634 mole
Amount of CL2 1.744
71 = 0.0246 mole
From equation: 2 moles of Au = 3 moles of Cl2
0.00634 mole of Au = 0.00634 x 3
2
= 0.00951 mole Cl2
Amount of Cl2 available = 0.0246 mole but amount required by all the
Au for the reaction = 0.00951
:. Cl2 is in excess
(iii) Excess amount of CL2 = amount available - amount used
= 0.0246 - 0.00951
= 0.015 mole
Alternative Method
(i) 2Au(s) + 3 Cl2(g) 2AuCl3(s)
(ii) 2 x 197g of Au = 3 x71g of Cl2
394g Au = 213g of Ci.,
1.2Sg = 213 x 1.25
394
= 0.675g of Cl2
1.25g of Au will require 0.675g of Cl2
:. Cl2 is in excess
(iii) Excess amount of Cl2 = amount available - amount used
= 1.744 - 0.675
= 1.069g



