Question 1A
1 A is a solution of 0.050 moldm-3 H2C204
B is a solution of KMnO4 (potassium tetraoxomanganate (VII), of unknown concentration.
(a) Put B into the burette. Pipette 20.0cm3 or 25.0cm3 of A into a conical flask and add about 10.0cm3 of dilute H2SO4. Heat the mixture to about 40oC-50oC and titrate it while still hot with B.
Repeat the titration to obtain consistent titre values.
Tabulate your results and calculate the average volume of B used.
Observation
The question is a redox titration. Majority of candidates were not familiar with the demands of the question, and the performance was fair.
In part (a), majority of the candidates were able to tabulate their burette readings. However, some candidates’ readings were not consistent and concordant.
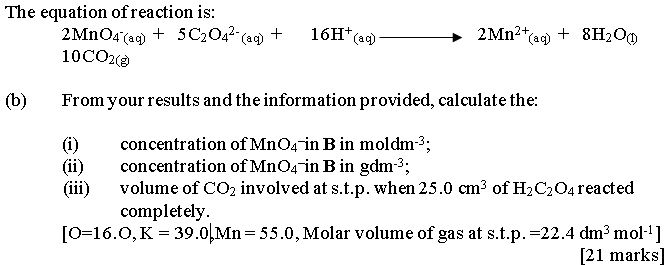
In part (b), some candidates mistook redox reaction for acid-based titration. Hence, they found it difficult to get the correct mole ratio for the reactants, and the molar concentration for KMnO4. Besides, majority of the candidates could not calculate correctly the volume of CO2 evolved
at s.t.p. when 25.O cm3 of H2C2O4 reacted completely.
Expected answers include:
(a) Two consistent/ concordant titres
Averaging