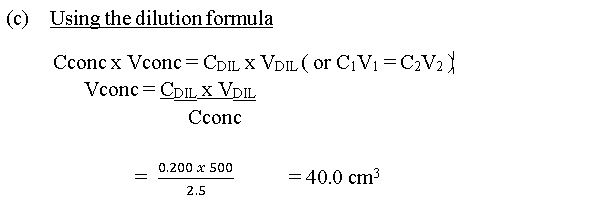Question 3A
3. (a) What difference in physical properties enable the separation of mixtures by:
(i) simple distillation;
(ii) paper chromatography;
(iii) fractional distillation. [3 marks]
(b) Give a reason for each of the following practices during titration in the laboratory.
(i) White tile is placed under the conical flask.
(ii) Burette readings are always recorded to two decimal places.
[2 marks]
(c) Calculate the volume of 2.5 moldm-3 stock HCl required to prepare 500 cm3 of 0.20 modm-3 HCl.
The question tested candidates’ familiarity with the practical activities suggested in their teaching syllabuses.
Observation
Majority of the candidates did not have knowledge of paper chromatography. They could not state a reason each for placing white tile under conical flask. and recording burette reading to 2 decimal places during titration. However, they showed understanding of dilution in stoichiometry and chemical reactions.
Expected answers include:
Question 3
(a) (i) Wide difference in boiling points of components (for miscible liquids) / Difference in physical states (for liquid – solid solution)
(ii) Difference in adsorption / retention rate of components
(iii) Close difference in boiling points of components
(b) (i) To make the end point colour change easily visible / in order not to overshoot the end point
(ii) To ensure (high) accuracy of the titre value