Many candidates were unable to state the uncertainty principle correctly. Some replaced the inequality sign with equality sign which rendered the equation wrong.
-34
At the substitution level, 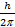 was wrongly used as was wrongly used as 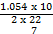 instead of 1.054 x 10-34. This is as a result of poor interpretation of the question. instead of 1.054 x 10-34. This is as a result of poor interpretation of the question.
Performance was poor.
The expected answer is Let DE = the uncertainty in determining the energy of the electron at the level.
DE. Dt ³ h/2p (the uncertainty principle)
OR
DE ³ h/2p 1/Dt
³ 1.054 x 10-37x 1/(2.0 x 10-9)
³ 5.27 x 10-28J
NOTE: Do not accept >,< or = in place of > |



