Question 12
-
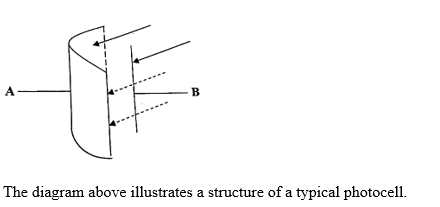
- Identify each of the parts labelled A and B.
- State one function each of A and B
- Einstein’s photoelectric equation can be written as E = hf – Wo. State what each of the terms E, hf and Wo represent.
- A photon is incident on a metal whose work function is 1.32 eV. An electron is emitted from the surface with a maximum kinetic energy of 1.97 eV. Calculate the frequency of the photon. [1 eV = 1.6 x 10-19 J]
-
- Define half-life of a radioactive element.
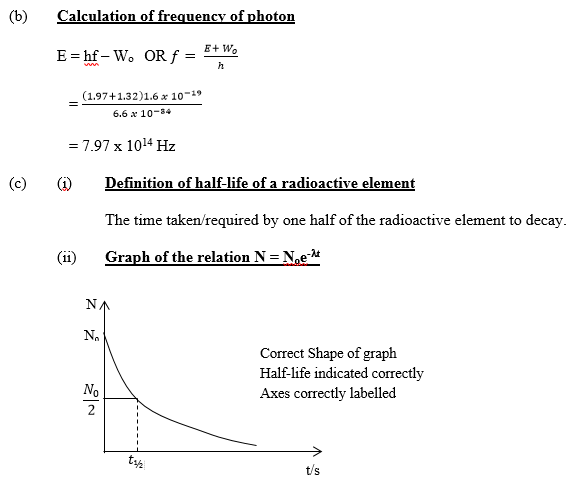
- Sketch a graph of the relation N = Noe-λt and indicate the half-life.
Observation
Part (ai): This question was popular among the candidates but unfortunately performance was
fair. Many candidates could not correctly identify the parts.
(ii) Performance was fair. Some candidates were unable to state the function of each part.
(iii) Performance was fair. Some candidates were unable to identify or state the terms
correctly.
Part (b): Performance was fair. Many candidates scored full mark in the calculation after
correctly substituting into the equation. A few that did not convert electron volt to joule
could not score beyond the equation in the formula.
Part (ci): Performance was fair. Many candidates got the definition of half-life correctly but a
few who defined half-life as “time taken by one half of a radioactive atom to decay” did not
score any mark.
(ii): Many candidates sketched the graph satisfactorily but a few were unable to indicate the
half-life.
The expected answer is
(a) (i) Identification of labelled parts of photocell
A - Emitter / cathode
B - Collector / anode
(ii) Function of part of photocell
A - emits electrons
B - attracts electrons
(iii) Meaning of terms in Einstein’s photoelectric equation
E - kinetic energy of photoelectrons
hf - energy of incident photon
Wo - work function of metal