Question 9
(a) (i) Explain latent heat.
(ii) State two factors that affect the rate of evaporation of a liquid
(b) Explain each of the following observations:
(i) On a dry day, water in a clay pot is cooler than water in a closed plastic container;
(ii) Food gets cooked faster in a pressure cooker than in an ordinary cooking pot.
(c) State two effects of heat on a substance.
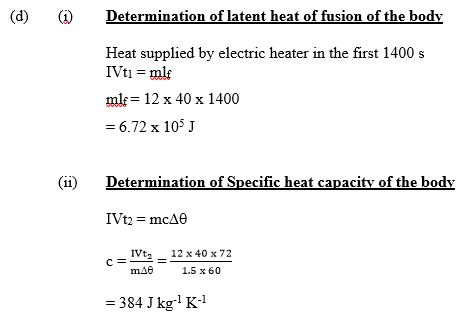
(d) A 40 V electric heater is used to supply a current of 12 A for 1400 s to a body of mass 1.5 kg at the melting point of the body. The body melts and its temperature rises through 60oC in an extra 72 s. Determine the:
(i) latent heat of fusion of the body;
(ii) specific heat capacity of the body.
Observation
Part (ai): This question was fairly attempted. A few candidates approached it the right way. A
good number defined it with additional information to score full mark. Those who merely defined
it lost some mark.
(ii) This part was well attempted. However, those who wrote “surface area of the liquid” instead
of exposed surface area” did not score.
Part (b): Performance was poor. Most candidates responded wrongly to the question signifying
complete ignorance of the principles of Physics involved in evaporation.
(ii) Most candidates were unable to use principles of Physics to explain the concepts.
Part (c): Performance was fair.
Part(di): Majority of candidates started correctly with the formula Ivt = ml but got it all
wrong in substitution.
(ii) Many candidates scored full mark. However, many candidates did not score beyond the formula
ivt = mcθ as they took time t to mean the sum of 1400s and 72s. The correct time in this segment
was 72s only.
The expected answer is
(a) (i) Explanation of latent heat
When a substance is changing state, it absorbs/gives out thermal/heat energy which does not
result in temperature change. This heat absorbed or given out during change of state is known as
latent heat.
(ii) Factors that affect the rate of evaporation of a liquid
- exposed surface area of the liquid
- temperature of the liquid
- wind / draught
- humidity / amount of water vapour
- nature of the liquid
- air pressure.
(b) Explanation of observations
(i) Water in a clay pot is cooler than water in a closed plastic container
The clay pot has pores which allow evaporation to take place. In the process of evaporation,
molecules take latent heat from the bulk of the water, leaving the water cooler; but the plastic
container does not have pores (to allow for evaporation).
(ii) Food gets cooked faster in a pressure cooker than in an ordinary cooking pot
With the pressure cooker, increased pressure raises the boiling point of water within a short
period. This leads to faster cooking. Ordinary cooking pot operates at normal atmospheric
pressure.
(c) Effects of heat on a substance
Change of/in:
- state
- temperature
- dimension
- colour
- electrical resistance