Question 1
(a)What is osmosis? [3 marks]
(b)
(i) With the aid of suitable diagrams, explain briefly how the process of osmosis could
be demonstrated in the laboratory using uncooked peeled yam tuber. [12 marks]
(ii) What will happen if the peeled yam tuber is replaced with boiled peeled yam? [1 mark]
(iii) Give one reason for the answer in 1(b)(ii). [2 marks]
(c) Name two other materials that could be used to replace the peeled yam tuber. [2 marks]
Observation
Some candidates could explain osmosis but some could not relate the process with specific reference to concentration of the solvent or solute. They could not clearly state the direction of movement of molecules. All most of them wrote was ‘semi permeable membrane’. In the diagram, most of the candidates did not use a control experiment; most of them only used one diagram. A few of them drew the diagram without labels.
In question 1 (b) (ii), some candidates did not know that boiling of the yam tuber will kill the living cell membrane that should have acted as the semi permeable membrane.
Many candidates could state other materials that could be used to carry out osmosis experiment.
The expected answers are:
(a)
Osmosis is the process by which water molecules; move from region/area of lower concentration of solute/weak/hypotonic/higher water molecules concentration; to a region/area of higher concentration of solute/strong/hypertonic/lower water molecules concentration; through a semi-permeable membrane.
(b)
(i) Demonstration of osmosis using peeled yam tuber
- The peeled yam tuber was cut transversely into two thick slices labelled A and B;
- A large cavity was made in the middle of each slice and the base of each was flattened;
- A strong salt/sugar solution was poured into the cavity of A; the level of the solution marked;
- An equal volume of water was poured into the cavity of B;
- And the level of the water was marked;
- Set up A and B were placed in a glass trough/beaker/container/basin containing water;
- And were both allowed to stand for 4-6/some/few hours;
- The set up B stands as the control;
- At the end the level of salt/sugar solution in A rose above the pin/mark/marked level;
- While the level of water in the container/basin/trough/beaker decreased;
- The level of water in B remained the same/ no water entered B;
- It can be concluded that osmosis took place in A with the yam tuber serving as a semi-permeable membrane.
Diagram
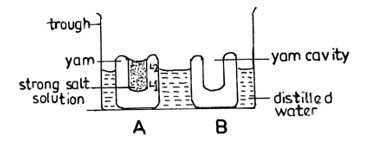
Labels: Peeled yam tissue, yam cavity, water/solute/weak salt solution, trough/beaker,basin/container, salt/sugar solution/strong solution.
Note: Spellings must be correct to score.
Details
Yam cavity shown (YC)
Different levels of solution/water shown (DL)
(ii) Use of boiled peeled yam
Osmosis will not take place/level of water will not increase.
(iii) Reason
The yam tissue/cells which serve(s) as the semi permeable membrane is destroyed/killed by boiling/destroyed by heating/boiling.
(c) Other materials that could be used to replace the peeled yam tuber
- Sweet/Irish potato tuber;
- Peeled cocoyam corm;
- Unripe plantain;
- Unripe pawpaw fruit;
- Pig’s bladder;
- Sheep’s bladder;
- Cellophane paper;
- Toad/frog skin;
- Fresh egg membrane.
Note: Spellings must be correct to score.
