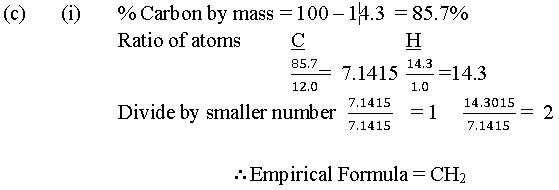Question 3
. (a) (i) Define structural isomerism.
(ii) State the class of alkanols to which each of the following compounds belongs:
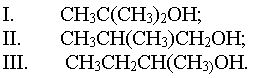
(b) (i) Write the formulae of the products formed in the following reactions:
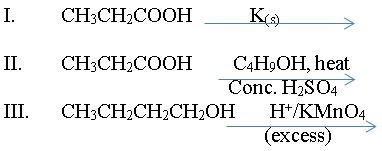
(ii) Name the major product(s) of each of the reactions in 3(b)(i).
Observation
The question was popular among the candidates, and majority of them responded to it.
In part (a), majority of the candidates correctly defined structural isomerism, but could not
classify the alkanols based on the position of the alkyl group on the structures.
In part (b), majority of the candidates could not write the correct formulae of products formed from the reactions.
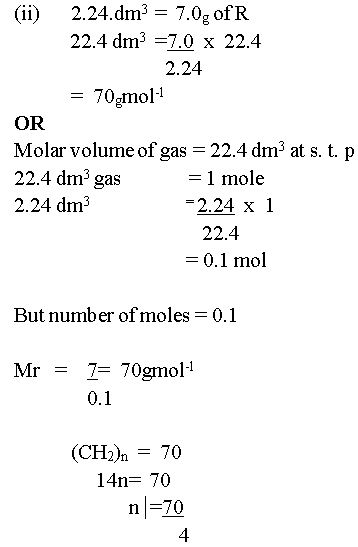
In part (c), few candidates could calculate the empirical formula, but majority could not calculate the molar mass of the hydrocarbon from the information provided.
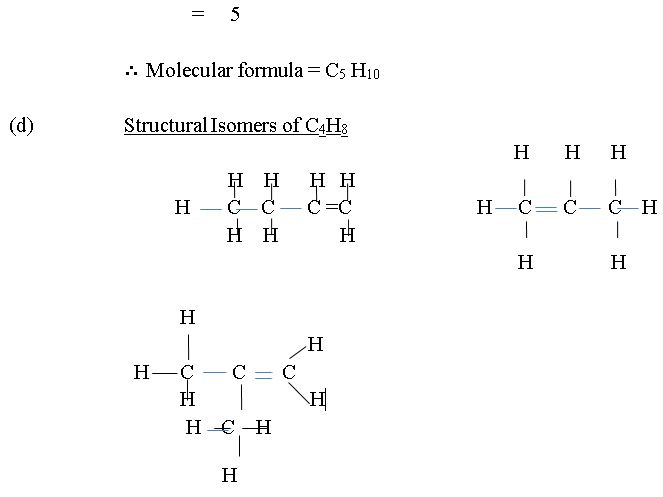
In part (d), majority of the candidates could not draw three isomers of alkene. Some drew one or two straight chain isomers.
The expected answers include:
(a) (i) The occurrence of /existence of/condition whereby compounds with the same
molecular formula have different structural formulae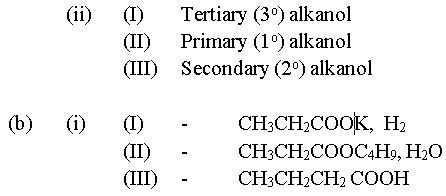
(ii) Major products in b(i)
(I) - potassium propanoate
(II) - Butyl propanoate
(III) - Butanoic acid