The question was attempted by majority of the candidates and the performance was
good.
In part (a)(i), candidates correctly gave name and corresponding nature of
the radiations emitted during radioactivity as follows:
- alpha particle, (doubly charged) helium nucleus/high ionizing/low penetrating
power/deflected towards negative plate
- beta particle, (fast moving) electron/moderate ionizing/moderate penetrating
power/deflected towards positive plate
- gamma radiation, (high energy) electromagnetic radiation/low ionizing/high
penetrating power/no effect on magnetic field.
Some candidates however lost marks because of spelling mistakes and use of
symbols to denote the particles (i.e alfa for alpha or a instead of alpha).
In part (a)(ii), only very few candidates were able to give corresponding differences
between chemical reaction and nuclear reaction as follows:
Chemical reaction |
|
Nuclear reaction |
- atoms are rearranged by the |
- |
elements are converted to other |
breaking and forming of chemical |
|
elements/new elements may be |
bond/no new atom is |
|
formed/atomic number or mass |
formed/atomic number or mass |
|
number of each element may change. |
| number of each element does not |
|
|
| change |
|
|
- only (valence) electrons are
involved. |
|
- protons, neutrons, electrons and other
elementary parties may be involved. |
- reactions are accompanied by
absorption/release of (relatively)
small amount of energy. |
|
- reactions are accompanied by release
of (relatively) large amount of energy. |
- rate of reaction is influenced by
temperature, pressure,
concentration and catalyst. |
|
- rate of reaction normally are not
affected by pressure, temperature and
catalyst. |
In(a)(iii), most candidates correctly balanced the nuclear reactions and identify X and Y
thus:
(iii) 21284PO → 20882Pb +42 X X is α- particle/helium nucleus I42He
13755Cs → 13756Ba + 0-1Y Y is β - particle/electron 0-1e
In part (b) (i) - (v), majority of the candidates correctly answered as follows:
(i) atomic number - 17
(ii) group 7
(iii) - formation of diatomic molecules
- formation of monovalent ions/same valence electrons
- they are coloured substances
- they are oxidizing agents
- most electronegative in its period/smallest atomic size in its period
- they are non metals
(iv) chlorine (formula was not accepted)
(v) 3Cl2 + 6NaOH → 5NaCl + NaCl03 + 3H20
However, some of the candidates lost marks because they could not give a correct balanced
equation for the reaction of element X and hot concentrated NaOH.
In part (c )(i), most candidates lost marks because they did not know that graphite was used
as a lubricant because of its planar layers which slides over each other and that diamond
has three dimensional network which makes it so hard hence, used as a cutting tool in I and
II respectively.
In part (c)(ii), candidates correctly wrote the reaction equation thus:
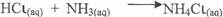

Also the gave the name of the reaction as combination/neutralization in (iii).



