Question 1B
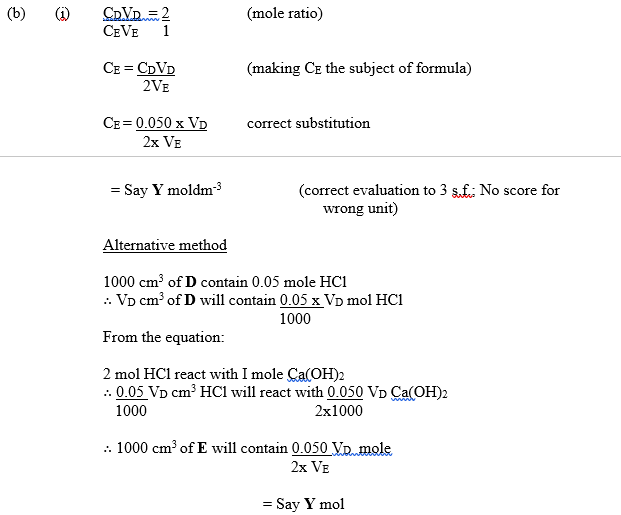
D is 0.050 mol dm-3 HCl.
E is a saturated solution of Ca(OH)2 at room temperature.
- Put D into the burette and titrate it against 20.0 cm3 or 25.0 cm3 portions of E using methyl orange as indicator. Repeat the titration to obtain concordant titre values.
Tabulate your results and calculate the average volume of D used.
The equation for the reaction is:
Ca(OH)2 + 2HCl → CaCl2 + 2H2O
- From your results and the information provided, calculate the solubility of Ca(OH)2 at room temperature in:
- moles per dm3 (mol dm-3);
- grammes per dm3 (g dm-3).
- What volume of distilled water must be added to 500 cm3 of E in order to reduce the concentration of ca2+ ions to one quarter of its original value?
[H = 1.0, O = 16.0, Ca = 40.0]
Observation
(a) Two concordant titres = 8 marks
Averaging = 2 marks