Question 9
-
(a) Define specific latent heat of vapourization.
(b) Why is the freezing compartment containing the evaporator normally located at the top of a fridge?
(c) Using the kinetic theory of matter, explain how addition of heat to a substance causes an increase in its temperature. 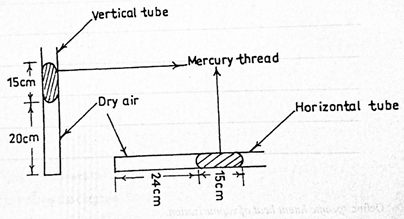
The diagram above illustrates two capillary tubes of uniform cross-sectional area containing air trapped by mercury. If Boyle’s law is obeyed, calculate the atmospheric pressure.
(e) Red beans of mass 100 g is capable is capable of releasing 240 kcal of energy. In a day, an adult requires 2200 kcal to perform optimally, calculate the:
(i) mass of beans required to sustain 500 adults;
(ii) mass of fuel that would be required if a nuclear reactor were to produce the same amount of energy as in 9(e)(i). [1 kcal = 4184 J; c = 3.0 x 108ms-1]
Observation
Part (a) Majority of the candidates attempted this question but few obtained the maximum scores. Some candidates missed out “unit mass” or “without a change in temperature” or “at boiling point” to score zero for an incorrect definition. Performance was fair.
Part (b)Majority of candidates proved that they lack knowledge of the operation of a refrigerator. Performance was poor.
Part (c) Most candidates mixed up kinetic theory with molecular theory in their explanation. Performance was poor.
Part (d) This was a popular question but some candidates were unable to interpret the problems with respect to Boyles’s law. Performance was below average.
Part (e) (i):Candidates performance was fair
(ii) Performance was fair.
The expected answer is:
(a) Specific Latent Heat of Vapourization
The quantity of heat required to change 1 kg/ unit mass of a substance from its liquid state at its boiling point/constant temperature to vapour.
(b) Reason Why Freezing Compartment Containing Evaporator is normally located at the top of a Fridge
Freezing compartment is located at the “top” of the fridge so that convection can be set up in the fridge. Cold air is denser than warm air, so it will sink and the warm air will rise.
(c) Explanation Using Kinetic Theory on Increase in Temperature
(Addition of heat to a substance) causes increase in the internal energy of the molecules. This increases the average kinetic energy resulting in a rise in temperature.
