Question 5
- (a) (i) List three physical properties of water.
(ii) Write a balanced chemical equation to show the effect of water on calcium.
[5 marks]
(b) (i) Draw and label a diagram for the laboratory preparation of a dry sample of sulphur (IV) oxide.
(ii) Write a balanced chemical equation for the reaction in 5(b)(i).
(iii) State the precaution that must be taken in the preparation of the gas.
[10 marks]
(c) (i) Consider the following gases:
HCl, H2, NH3, SO2, O2
State which of the gases:
- Is an acid anhydride;
- Could be used to demonstrate the fountain experiment.
(ii) Give two examples of gaseous fuel.
(iii) Give reasons why water gas is a better fuel than producer gas. [7 marks]
(d) State how extraction of iron using the blast furnace has harmed the environment and society. [3 marks]
Observation
This question was attempted by majority of the candidates and their performance was above average.
In part (a), majority of the candidates listed three physical properties of water.
In part (b), majority of the candidates drew and label a diagram for the laboratory preparation of a dry sample of sulphur (IV) oxide.
In part (c), majority of the candidates stated the gases which are acid anhydride and could be used to demonstrate fountain experiment.
In part (d), majority of the candidates correctly stated how the extraction of iron using the blast furnace has harmed the environment.
The expected answers include:
(a) (i) - odourless
- colourless
- has boiling point of 100oC / a freezing point of OoC
- has a maximum density of 1 g cm-3 at 4oC
- tasteless / insipid taste
(ii) - Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
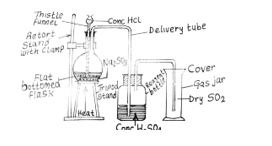
(ii) Na2SO3(aq) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + SO2(g)
K2SO3 + 2HCl → 2KCl + H2O + SO2
Na2SO3 + H2SO4 → Na2SO4 + H2O + SO2
K2SO3 + H2SO4 → K2SO4 + H2O + SO2
(iii) must be prepared in a fume chamber / cupboard
(c) (i) I. SO2
II. HCl , NH3
(ii) - water gas
- coal gas
- producer gas
- butane
- methane / natural gas
- hydrogen
- synthesis gas
- biogas
- CO
(iii) Constituents of water gas (CO and H2) are both combustible and give a high
caloric / heat value than producer gas whose constituent (CO and N2)
gases are not all combustible.
(d) - causes air pollution
- causes water pollution / water related health problems
- excessive heat to the environment / global warming
- soil pollution
