Question 1
In a volumetric analysis, 24.80 cm3 of 0.100 mol dm-3 of a mineral acid, Y, neutralized 25.0 cm3 of an alkaline solution containing 5.80 g of Na2CO3 per dm3.
(a) From the information provided, calculate the:
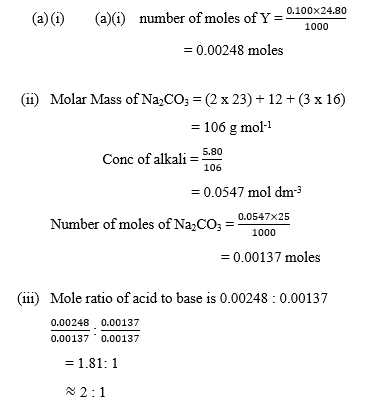
(i) number of moles of Y;
(ii) number of moles of Na2CO3;
(iii) mole ratio of the acid to base in the analysis.
(b) (i) What is the basicity of Y;
(ii) Suggest what Y is likely to be;
(iii) Give a reason for the answer suggested in 1(b)(ii).
[Na = 23, O = 16.0, C = 12.0]
(c) In a titration, 23.40 cm3 of A containing 15g of CH3COOH per dm3 reacted with
25.0 cm3 of solution B, containing x g of Na2CO3 per dm3.
The equation for the reaction is:

From the information provided, calculate the:
(i) concentration of A in mol dm-3;
(ii) concentration of B in mol dm-3;
(iii) value of x.
[ H = 1.0, C = 12.0, O = 16.0, Na = 23.0]
[22 marks]
Observation
This question was compulsory for candidates. Their performance was below average
In part (a), majority of the candidates could not calculate the number of moles of Y and number of moles of Na2CO3.
In part (b), majority of the candidates could not state the basicity and suggest what Y could be.
In part (c), majority of the candidates were able to calculate the concentration of A and B in moldm-3. They also determined the value of x in the compound.
The expected answers include:
(b)(i) Its basicity is one (to score mole ratio must be 2 : 1 in a (iii))
(ii) Y could be HCl/HNO3 (Do not accept any monobasic organic acid) (to score basicity
must be)
(iii) Y contains one ionizable hydrogen atom per molecule (to score, basicity must be
correct)

