Generally, candidates’ responses fell short of expectation in part (a) but performance was very high in parts (b) & (d). Majority of candidates did not answer part (c) of the question. The few that gave responses to it did more of guess work than state the required general conclusion.
The expected answers were as follows:
(a) A clinical thermometer has small temperature range. The glass will crack/burst due to excessive pressure created by expansion of mercury.
(b) Increase in pressure
(i) increases the boiling point;
(ii) decreases the melting point.
- At the boiling point of pure water, the saturated vapour pressure (s.v.p)
of the water is equal to the external atmospheric pressure.
(d) Diagram
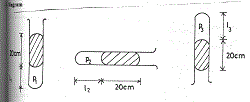
Volume V is proportional to length Ɩ stated or implied
(i) P1V1 = P2 V2 implies P1 Ɩ1 = P2Ɩ2
(76 + 20) 15 = 76 x Ɩ2
Ɩ2 = 96 x 15
76
Ɩ2 = 18.95 cm
(ii) P1 Ɩ1 = P3 Ɩ3
(76 + 20) 15 = (76 – 20) x Ɩ3
Ɩ3 = 96 x 15
56
Ɩ3 = 25.7l cm



