Candidates generally exhibited lack of knowledge of emission and absorption line spectra.
Many candidates presented complicated but meaningless diagrams of the photocell. However, majority of the candidates had no problem responding satisfactorily to parts (c) and (d).
The expected answers are as follows:
(a)(i) Emission line spectra consist of distinct and separate bright lines of definite wavelengths on a dark background.
Any valid additional information e.g
- They are obtained when light from a luminous source undergoes dispersion and is
observed directly.
- When an electron moves from one energy level to another energy level, line spectra are observed
- The colour of the spectra is a characteristic of the source.
(ii) Line absorption spectra are obtained when light passes through a cool gas and certain wavelengths of the light are absorbed. This gives series of dark lines, each corresponding to one of the wavelengths absorbed.
(b) Diagram
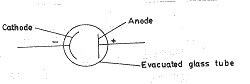
When radiation of appropriate frequency falls on the cathode (with photosensitive surface) electrons are emitted. Because the anode is positive with respect to the cathode, the electrons are attracted to the anode. The electrons flow completes the circuit and current flows.
(c)(i) Wave properties of X-rays
- diffraction
- interference
- reflection
- refraction
- polarization
(ii) Uses of X-rays other than in medicine
- to study crystal structures
- to detect presence of cracks in welded parts
- in artistic works for determining the authenticity of such works
- at security check points to scan
- X-ray spectroscopy for identifying isotopes of elements.
(d)

ΔE = Ei - Ef
= - 1.6 – (- 10.4)
= 8.8 e V or 1.41 x 10-18 J
E = hf = hc/λ
or
λ = hc/E
= 6. 6 x 10-34 x 3 x108

8.8 x 1.6 x 10-19
λ = 1.4 x 10-7 m



